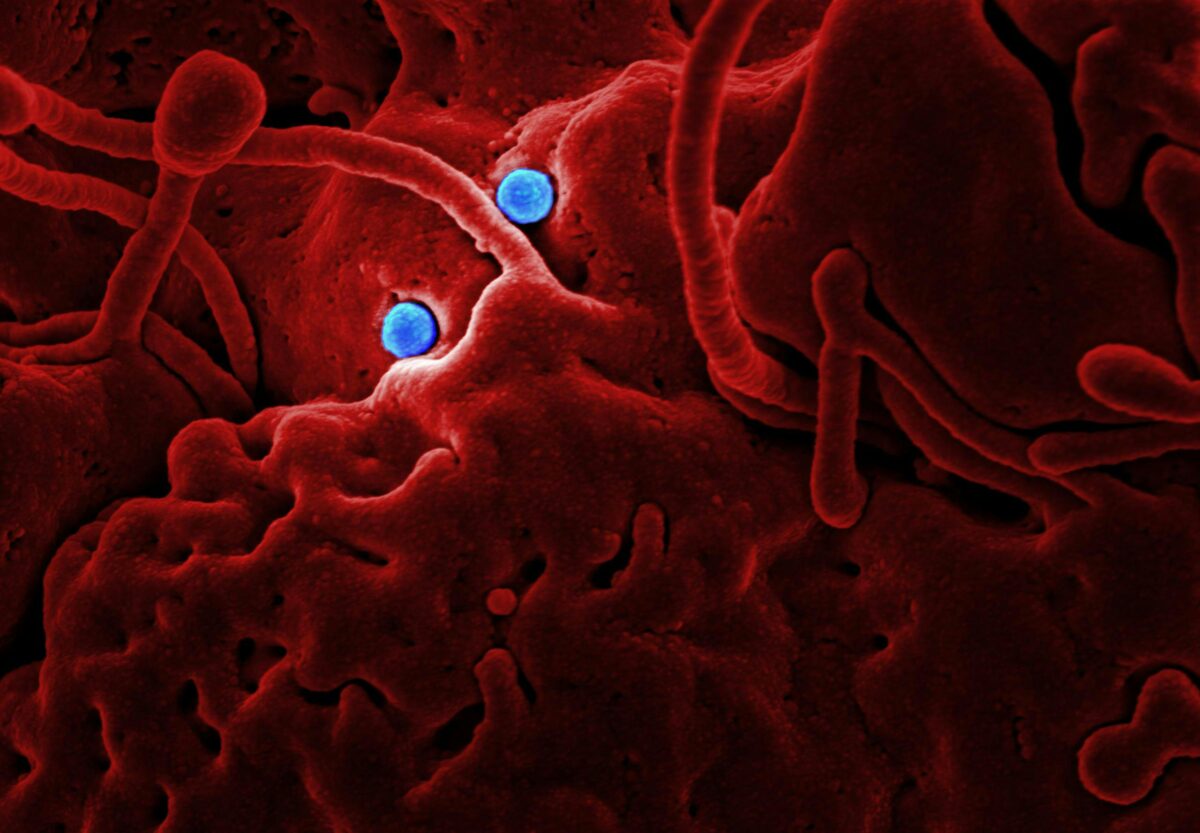
How do harmful bacteria like Salmonella find their way through the human body to cause infection? The human gut is a complex and crowded place, home to over 100 trillion beneficial microbes that form a protective barrier against invaders. In this vast, microscopic metropolis, a single Salmonella bacterium faces overwhelming odds, like trying to find one specific building in a city of millions while being outnumbered a million to one.
For years, scientists believed these pathogens primarily used a process called chemotaxis—which is like a sense of smell for bacteria—to find their way around. They follow chemical trails, moving toward or away from certain compounds to navigate. But a groundbreaking new study from a team of researchers at UC Davis Health, published in Nature Microbiology, suggests there’s a much more electrifying story at play.
The study’s provocative discovery is that a novel bioelectrical mechanism, not just chemical cues, helps pathogens target vulnerable entry points in the gut. Essentially, your gut has an internal electrical wiring system, and harmful bacteria have learned how to hack it. This discovery could revolutionize our understanding of how infections take root and potentially lead to new strategies for treating and preventing common illnesses like food poisoning.
The discovery flips the script on what was previously known about how bacteria navigate the body. The research showed that while harmless E. coli are repelled by these electrical fields, Salmonella is actively drawn to them through a process called galvanotaxis. It’s as if the gut’s defenses have a hidden weakness that Salmonella is perfectly designed to exploit.
How Salmonella Sneak In
The human gut is not a uniform landscape. It has different types of cells with specialized jobs. The majority of the gut lining is made up of villus epithelium, which are tiny, finger-like projections responsible for absorbing nutrients. But scattered throughout are smaller, dome-shaped structures called follicle-associated epithelium (FAE), which contain specialized cells called microfold, or “M” cells. These M cells act as a gateway for the immune system to sample bacteria and antigens, but they are also the precise entry points that pathogens like Salmonella use to invade the body.
The research team set out to understand how Salmonella finds this tiny, vulnerable entry point when it’s surrounded by a massive population of harmless bacteria. The team developed a novel ex vivo mouse model, which means they used a piece of mouse gut tissue, keeping it alive in a lab setting to observe it. When they introduced both Salmonella and a common, harmless strain of E. coli to the tissue, the results were striking: the E. coli preferred to stay in the villus regions, while the Salmonella accumulated heavily in the FAE areas.
The researchers used a special tool called a vibrating probe to measure the electrical currents in the gut tissue. The results were eye-opening: the FAE areas had a distinct electrical signature, a sustained outward current, while the surrounding villi had a different signature, a sustained inward current. The scientists discovered that a protein called the cystic fibrosis transmembrane conductance regulator (CFTR) was responsible for generating these electrical currents. CFTR regulates the flow of chloride ions across cell membranes and is well-known for its role in cystic fibrosis.
The New ‘Arms Race’ Against Bacteria
To confirm the role of this electrical field, the researchers performed a key experiment. When they used a drug to block the CFTR protein and disrupt the electrical currents, the Salmonella‘s attraction to the FAE significantly decreased. This was a powerful piece of evidence showing that the electrical currents generated by the CFTR protein are a major factor in guiding bacteria to specific locations in the gut.
To further validate their findings, the researchers created their own electrical fields in a lab dish and observed the bacteria. They found that Salmonella and E. coli responded in opposite ways to the same electrical cue. E. coli swam toward the positive end of the field (the anode), while Salmonella swam toward the negative end (the cathode). The electrical fields even made the bacteria swim faster. This confirmed that the electrical currents, not just chemical signals, were steering the bacteria’s movements.
The study’s findings have far-reaching implications, not just for infectious diseases but for other chronic gut conditions like inflammatory bowel disease (IBD). For years, IBD has been linked to an abnormal immune response to good bacteria in the gut. Now, scientists can explore whether a person’s predisposition to IBD could be connected to abnormal bioelectrical activity in their gut lining. The study opens the door to a new “arms race” between medicine and pathogens, where we can begin to find ways to manipulate these electrical currents to prevent infections and maintain a healthy gut.
This research proves that the old saying about birds of a feather flocking together might need an update for the microbial world. In the crowded and competitive landscape of the human gut, Salmonella and E. coli aren’t just finding their way by scent. They are literally following different electrical currents, with one choosing a path that leads to infection while the other stays on a route that keeps us healthy.
Paper Summary
Methodology
The research was conducted using an ex vivo mouse caecum model, which means a piece of mouse gut tissue was kept alive in a lab setting to observe it. Researchers used a vibrating probe to measure the electrical currents in different gut areas. They also used pharmacological drugs to block specific ion channels, like the CFTR protein, to confirm its role in bacterial movement. Separate lab experiments were performed where bacteria were placed in an artificially created electrical field to observe their movements. The study also used competitive assays between wild-type and chemotaxis-deficient mutants.
Results
The study found that Salmonella specifically accumulated in the FAE regions, while E. coli preferred the surrounding villi. This localization was found to be independent of chemotaxis. Electrical measurements showed that the FAE and villi have distinct electrical potentials. The outward electrical currents in the FAE were generated by the flow of chloride ions regulated by the CFTR protein. When CFTR was inhibited, the Salmonella attraction to the FAE decreased, while E. coli recruitment to the FAE increased. In lab experiments, Salmonella migrated toward the negative end (cathode), while E. coli moved toward the positive end (anode) of the electrical field.
Limitations
The study was conducted on an ex vivo mouse model, which may not perfectly replicate the complex environment of the human gut. The study’s key measurements were also based on a limited number of mice.
Funding and Disclosures
The research was supported by grants from the National Institutes of Health (NIH), the Defence Advanced Research Projects Agency (DARPA), and the Fundação para a Ciência e Tecnologia (FCT).
Publication Information
The study, titled “Gut epithelial electrical cues drive differential localization of enterobacteria,” was published online on August 20, 2024, in the journal Nature Microbiology. The DOI for the paper is https://doi.org/10.1038/s41564-024-01778-8.