There are ever more urgent efforts to contain the rising costs of healthcare, without compromising the quality of care. Screening for cancer is one of the aspects of healthcare where there’s no room for error. Colorectal cancer (CRC) is the fifth most common cancer in the United States, and it is being diagnosed in younger and younger individuals. The current recommendation by the U.S. Preventive Services Task Force (USPSTF) is to begin colorectal cancer screening at age 45.
National guidelines include the use of a noninvasive screening test for early-stage CRC. There are two noninvasive tests available. One is a fecal immunochemical test (FIT). The other is a more expensive test which detects DNA, called Cologuard. The tests are equally effective at detecting adenoma versus CRC. Many studies have affirmed that FIT is more cost effective than other types of noninvasive colorectal cancer screening tests. It costs one-fifth as much as the DNA test.
Dr. Pavan K. Rao, a general surgery resident at Allegheny Health Network in Pittsburgh, performed a study looking at 91,297 people who had noninvasive screening with either FIT or the DNA test instead of a colonoscopy.
The annual costs for the tests: $1.1 million for a FIT, or about $24 per test, and $5.6 million for mt-sDNA, or about $121 per test. Costs were calculated using Medicare reimbursement rates. The researchers determined that transitioning all noninvasive colorectal cancer screening to FIT would result in annual savings of $3.9 million savings in the study population.
“Despite national guidelines suggesting that FIT be used as the primary noninvasive screening modality, we found that on review of our insurer’s claims data, a significant proportion of patients still receive a more expensive alternative test. There is substantial cost savings not only to our patients but to our health system with promoting appropriate use of noninvasive testing,” says Dr. Rao.
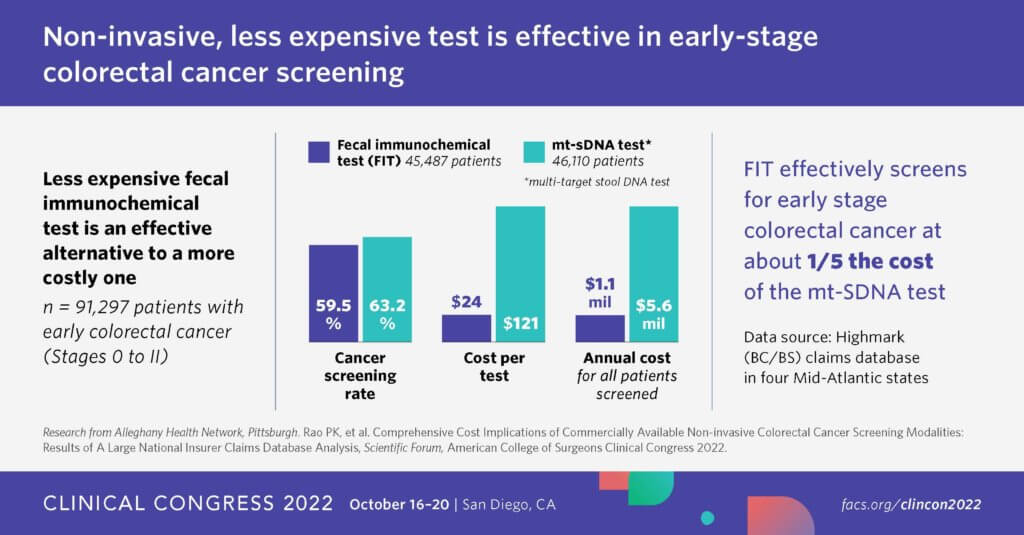
“There was no difference in the clinical stage at the time of diagnosis between the two tests, which again demonstrates the clinical quality maintained by switching to FIT,” Rao notes, on the variation between the two tests. “When you look at the national data for which the guidelines put forward, they found no difference between the two tests at detecting adenoma versus colorectal malignancy.
“In the current state of healthcare, we are thinking ever more about efficiency and reduction in costs while maintaining patient outcomes, and not compromising the quality of care we provide,” Rao continues. “I think a colorectal surgeon or any specialist who sees appropriate patients for colorectal cancer screening can use this data to provide recommendations of alternative screening tests to patients who primarily do not want to undergo colonoscopy. We cannot only say it is appropriate from a guideline standpoint, but we’re also reducing wasteful spending in health care by appropriately using the FIT.”
This research was presented at the Clinical Congress 2022 of American College of Surgeons.
