Subheading: Fruit Fly Study Provides Insight into Immune System Evolution and Human Susceptibility to Infections
Groundbreaking research reveals a fascinating discovery about the immune systems of fruit flies and their response to common bacteria found in their food and environment. Contrary to previous theories that suggested antimicrobial peptides, acting as natural antibiotics, had a general role in fighting a range of bacteria, this new study shows that specific genes are developed in the immune systems of fruit flies to combat bacteria commonly encountered in their surroundings.
Dr. Mark Hanson, from the Centre for Ecology and Conservation at the University of Exeter, explained the implications of the study. “We know that an animal’s food and environment determine the bacteria it encounters,” Dr. Hanson said in a statement. “This, in turn, shapes its ‘microbiome’ – the collection of microbes that live in and on its body – and our study shows how immune systems evolve in response to this, to control common bacteria that could otherwise cause harm. In immune terms, it proves the saying ‘you are what you eat’ – the flies’ immune systems contain peptides with remarkably specific functions for controlling common bacteria.”
One such bacterium, Acetobacter, commonly found in the fruits that flies and humans consume, can harm flies if it escapes the gut and enters the bloodstream. The researchers discovered that various fly species possess a specific peptide (Diptericin B) to control Acetobacter effectively.
“This peptide is the silver bullet that kills this specific bacterium,” Dr. Hanson elaborated. “Without it, flies are extremely vulnerable because Acetobacter is so common in rotten fruit.”
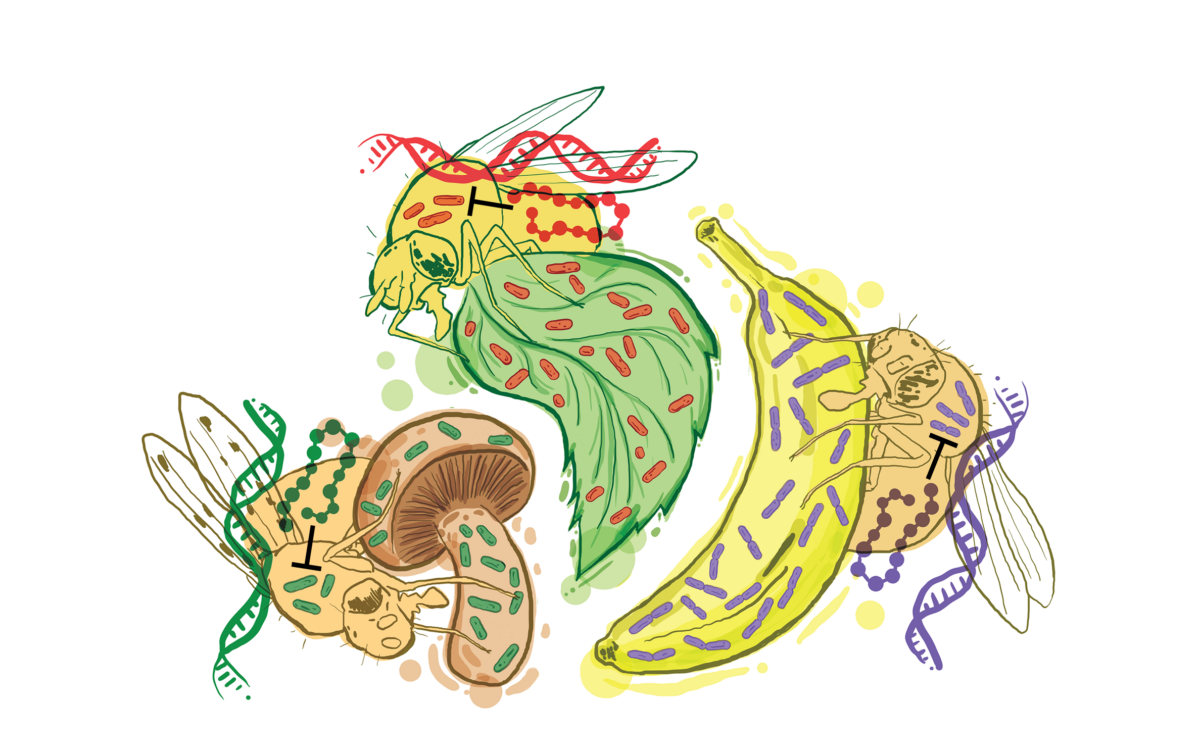
The study also uncovered evidence of “convergent evolution,” where different species develop similar responses to environmental challenges. Despite diverging from a common ancestor around 100 million years ago, the fly species in the study both evolved a Diptericin B peptide to control Acetobacter. Interestingly, closely related fly species that do not feed on fruit have lost their Diptericin B peptides over time, as Acetobacter is no longer prevalent in their environment.
Dr. Hanson believes that this evolutionary process might offer insights into human susceptibility to certain infections. “The way our bodies fight infections is really complex. But this sort of research helps us to view our immune system in a new light,” he said. “I hope it gets us to ask why our immune system is made the way it is. That can help us fight infections, including infections that resist antibiotics. Studies like this produce fundamental observations about life, and in turn, these can have crucial applications in the world around us.”
Funded by the Swiss National Science Foundation and Novartis Foundation, the research not only sheds light on the fascinating immune system evolution in fruit flies but also carries significant implications for human health and our understanding of infections. As scientists continue to explore the intricate relationship between the microbiome and immune systems, this research may offer crucial insights to develop innovative approaches to combat infections, including those that prove resistant to antibiotics.
The study is published in the journal Science.
