The most common symptoms of Covid-19 are loss of taste and/or smell and difficulty breathing. Yet, 60% of infected patients have gastrointestinal symptoms (GIS) such as nausea, diarrhea, and stomach pain. It is difficult to determine the actual effects COVID has on the gut because the interactions between the intestinal tissue and the virus are hard to study in humans. Since animals do not fully reflect how human organs are affected by infection, research has been limited. However, a novel tool called the Intestine Chip may have solved this problem, according to a recent study.
The Tool
Scientists from the Wyss Institute for Biologically Inspired Engineering at Harvard University and other Wyss organizations recently used the previously developed chip in tests to study coronavirus infection and potential treatments. The device is about the size of a USB memory stick. It is made of a clear, flexible polymer and has two parallel channels running through it: one lined with human blood vessel cells, the other with human intestinal lining cells. There’s a permeable membrane between the two channels that is designed to mimic digestion, whereby substances are delivered into the blood via the gut.
Testing the Device
The chip was infected via the human intestinal cell with a coronavirus that causes the common cold, then treated with nafamostat and remdesivir. The signs of infection were clear: compromised connections between the gut cells and cell damage/detachment in the blood vessel channels. The findings reveal that the nafamostat treatment significantly reduces the infection, but it does not restore the connections between the cells. Surprisingly, the more familiar remdesivir does not reduce the virus in the Intestine Chip. In fact, it actually damages the blood vessel channel cells by almost completely detaching them from the channel wall.
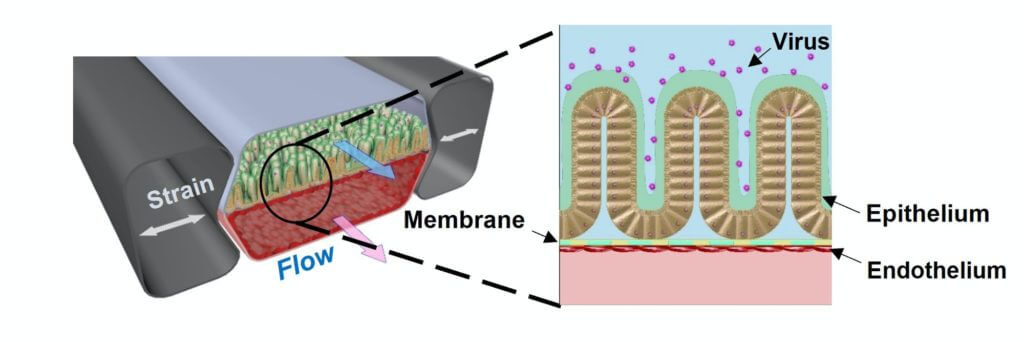
“We were surprised that remdesivir displayed such clear toxicity to the vascular tissue in the Intestine Chip. GI symptoms have been previously reported in clinical trials of remdesivir, and this model now gives us a window into the underlying causes of those symptoms. It could also help us better understand the efficacy and toxicity of other similar drugs,” says Girija Goyal, Ph.D., co-first author, and Senior Research Scientist at the Wyss Institute, in a recent statement.
Though preclinical, this new model could help identify drugs that target GI symptoms resulting from the common cold and coronavirus infections.
More Discoveries and Treatments
Since tests using the human intestinal cell confirmed that the Intestine Chip successfully models interactions between the gut, viruses, and treatments, the team tested oral drugs that have proven to effectively reduce infection by SARS-CoV-2 and other viruses in vitro. This time, human immune cells were introduced into the blood vessel channel of the device and the chip was pre-treated with nafamostat. These results show that more damaged cells attach themselves to the blood vessel wall. Also, this infection caused more inflammatory cytokines to be produced. These signal the body to send more immune cells to the site of the infection. The pre-treatment did increase the production of Lipocalin-2 – an antimicrobial protein – which could play a role in how cells respond to coronavirus infections.
“This study demonstrates that we can explore complex interactions between cells, pathogens, and drugs in the human intestine using our Intestine Chip as a preclinical model. We hope it proves useful in the ongoing effort to better understand the effects of SARS-CoV-2 and to identify drugs that could be used to combat future viral pandemics,” says senior author and Wyss Founding Director Don Ingber, M.D., Ph.D. Dr. Ingber is the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and a Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
The study can be found in Frontiers in Pharmacology.